Read the latest
Find out what’s new with ENDRA, TAEUS® and the future of medical imaging.
We’re changing the way clinicians measure fat in the liver, making it easier, less expensive, and more accessible for everyone.

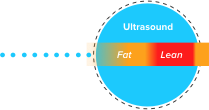
TAEUS® is a multi-featured Thermo-Acoustic device which uses a radio frequency (RF) source with ultrasound reception for enhanced tissue penetration, measuring liver fat.
It is CE-marked and commercially available in Europe.
Learn how ENDRA is making difficult-to-diagnose diseases easier to find and treat.
WATCH THE VIDEO >TAEUS® combines a pulsed radio frequency (RF) source and an RF applicator to deliver energy efficiently into the tissue. Some of the energy is absorbed and converted into an acoustic response, which is recorded by an integrated ultrasound acoustic receiver.
ENDRA is the first to use the unique combination of RF and ultrasound to produce meaningful data quickly, efficiently, and cost-effectively.
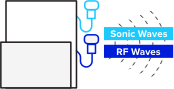
Use a traditional B-mode ultrasound to determine the region for fatty liver tissue assessment on the patient.
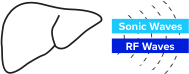
Use TAEUS® to pulse RF waves into the target region.
Energy creates small acoustic waves that represent a unique signature for lean and fat tissues.

Acoustic waves are detected by the TAEUS® integrated ultrasound acoustic receiver.
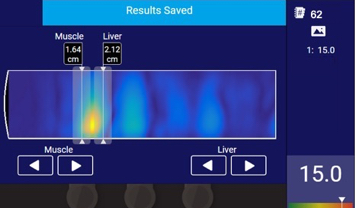
TAEUS® quantitative data is translated and displayed into clinically useful metrics.
“ENDRA’s TAEUS® technology could be a game-changer for the clinical care cycle of liver [and other] disease—from screening to diagnosis to therapy guidance.”
Find out what’s new with ENDRA, TAEUS® and the future of medical imaging.
ENDRA CLINICAL ABSTRACT: EUROPEAN ASSOCIATION FOR THE STUDY OF THE LIVER (EASL), STEATOTIC LIVER DISEASE (SLD) SUMMIT, SEPT 2023
ENDRA CLINICAL ABSTRACT: EUROPEAN ASSOCIATION FOR THE STUDY OF THE LIVER (EASL), INTERNATIONAL LIVER CONGRESS, JUNE 2023

ENDRA operates a Quality Management System which complies with the requirements of ISO 13485:2016 & EN ISO 13485:2016 for the following scope:
Certificate number: MD 697226
Effective Date: 2025-04-02
Expiry Date: 2028-04-01